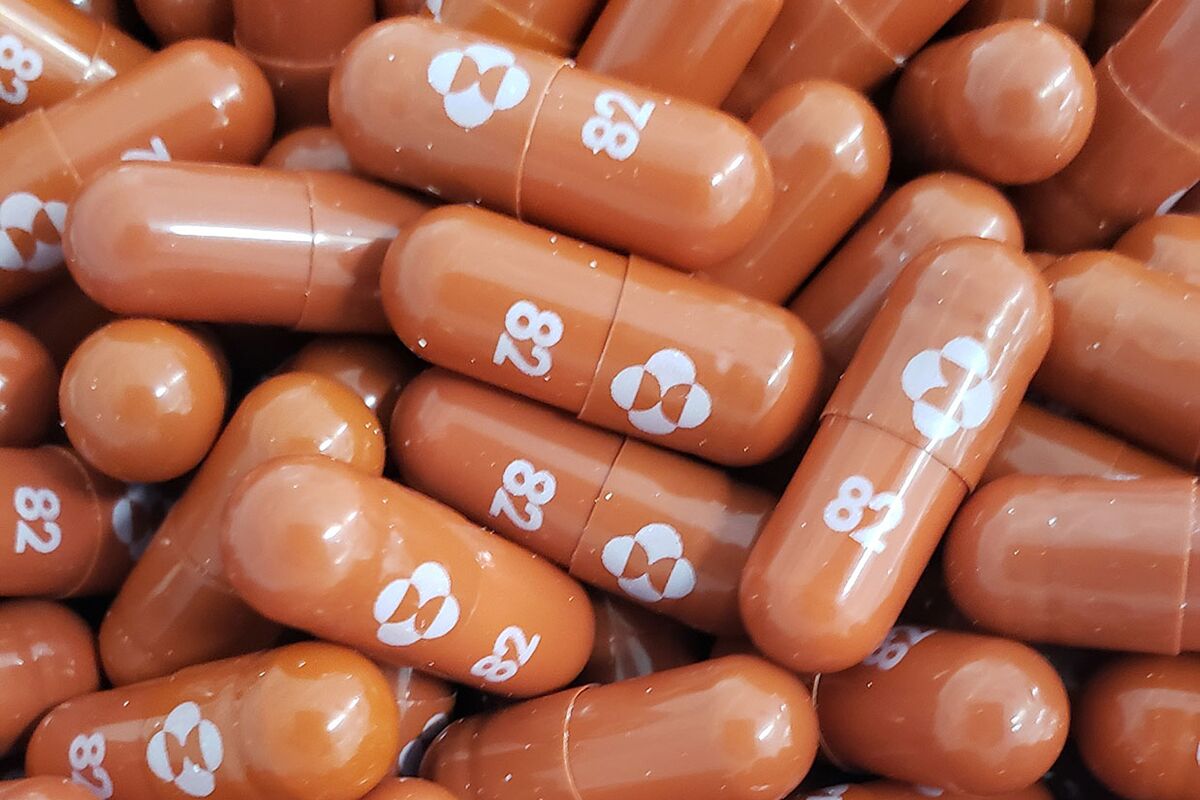
Merck & Co.’s Covid-19 antiviral pill molnupiravir reduced the risk of hospitalization or death by 50% in an interim analysis of a late-stage trial, findings that could give doctors another potent virus-fighting tool.
The drug giant and partner Ridgeback Biotherapeutics LP are halting the study and plan to seek an emergency-use authorization from the U.S. Food and Drug Administration as quickly as possible, Merck Chief Executive Officer Rob Davis said in an interview. Merck also plans to submit applications to regulators in other countries.
“We couldn’t be more thrilled with the results,” Davis said. “You don’t have to go to the hospital, you don’t have to go to a center to have it infused. It’s a pill you can take at home.”
Merck shares jumped 4.9% in trading before markets opened in New York.
Molnupiravir is intended for use in nonhospitalized Covid-19 patients who have had symptoms for five or fewer days and are at risk for a severe infection. The companies detailed the study analysis in a statement on Friday.
The clinical trial had been expected to conclude in November. The decision to stop the research was made at the recommendation of an independent committee and in collaboration with the FDA, Davis said.
If authorized, molnupiravir could become an important new tool for the treatment of Covid-19. While drugs known as monoclonal antibodies are available to stave off more severe infections, they can be difficult to administer, and demand for them has surged during the recent spike in virus cases fueled by the delta variant.
Adding molnupiravir would give doctors a much simpler option to treat infected patients that could keep them from filling strained hospitals. Delta’s rise has taxed health systems across the country, especially in areas where vaccination rates are lower than average.
Planned Analysis
The findings came out of a previously planned analysis of 775 patients who had been in the clinical trial since at least early August.
About 7% of the patients on molnupiravir went on to be hospitalized or die, versus 14% of those on a placebo. None of those taking molnupiravir died, compared with eight deaths among the placebo group.
The trial had planned to enroll 1,550 participants, and around 90% had already signed up. Patients who were taking a placebo will have the opportunity to start on molnupiravir, Davis said.
Merck said in the statement that it expects to produce 10 million courses of treatment by year-end, with more expected in 2022.
Earlier this week, Merck presented other data from early-stage research showing that molnupiravir appears to inhibit several major variants of the coronavirus, including the highly contagious delta strain.
 www.bloomberg.com
www.bloomberg.com
The drug giant and partner Ridgeback Biotherapeutics LP are halting the study and plan to seek an emergency-use authorization from the U.S. Food and Drug Administration as quickly as possible, Merck Chief Executive Officer Rob Davis said in an interview. Merck also plans to submit applications to regulators in other countries.
“We couldn’t be more thrilled with the results,” Davis said. “You don’t have to go to the hospital, you don’t have to go to a center to have it infused. It’s a pill you can take at home.”
Merck shares jumped 4.9% in trading before markets opened in New York.
Molnupiravir is intended for use in nonhospitalized Covid-19 patients who have had symptoms for five or fewer days and are at risk for a severe infection. The companies detailed the study analysis in a statement on Friday.
The clinical trial had been expected to conclude in November. The decision to stop the research was made at the recommendation of an independent committee and in collaboration with the FDA, Davis said.
If authorized, molnupiravir could become an important new tool for the treatment of Covid-19. While drugs known as monoclonal antibodies are available to stave off more severe infections, they can be difficult to administer, and demand for them has surged during the recent spike in virus cases fueled by the delta variant.
Adding molnupiravir would give doctors a much simpler option to treat infected patients that could keep them from filling strained hospitals. Delta’s rise has taxed health systems across the country, especially in areas where vaccination rates are lower than average.
Planned Analysis
The findings came out of a previously planned analysis of 775 patients who had been in the clinical trial since at least early August.
About 7% of the patients on molnupiravir went on to be hospitalized or die, versus 14% of those on a placebo. None of those taking molnupiravir died, compared with eight deaths among the placebo group.
The trial had planned to enroll 1,550 participants, and around 90% had already signed up. Patients who were taking a placebo will have the opportunity to start on molnupiravir, Davis said.
Merck said in the statement that it expects to produce 10 million courses of treatment by year-end, with more expected in 2022.
Earlier this week, Merck presented other data from early-stage research showing that molnupiravir appears to inhibit several major variants of the coronavirus, including the highly contagious delta strain.

Merck Surges as Covid Pill Looks to Ease Hospital Strains
Merck & Co. shares posted their biggest gain in five years after the company’s experimental pill slashed the risk of getting seriously ill or dying from Covid-19 in a study, findings that could eventually yield a simple way to treat many virus patients before they ever reach the hospital.






